What you need to know
Stem cells are the basis of a new type of medicine that is to use the unique properties of these cells to repair tissues or organs damaged by diseases or injuries. One speaks then of cell therapy or regenerative medicine.
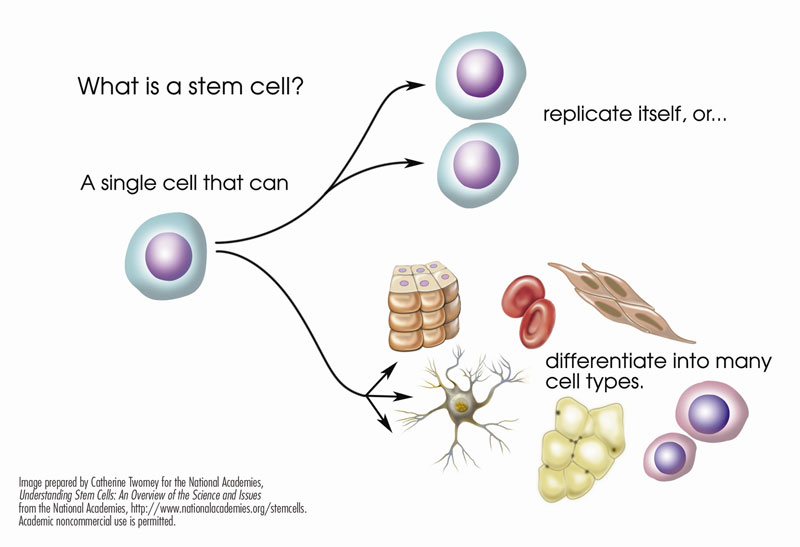
The power of stem cells
Cell therapy involves the use of pluripotent stem cells. Through a process called differentiation, these cells can turn into different types of specialized cells, such as red blood cells, white blood cells, platelets, eye cells, heart muscle cells, or nerve cells. These specialized cells can then repair damaged or diseased tissue.
The medicine of the future... today!
Regenerative medicine is one of our best hopes for the medicine of the future, as drug therapies generally only address symptoms and have to be maintained over the long term. Regenerative medicine tackles the cause of disease and provides hope for full recovery. In the long term, these therapies will improve our health and quality of life and provide considerable savings to the health care system.
What diseases can be treated with cell therapy?
Regenerative medicine has mainly been used in hematology, particularly to treat blood cancers such as leukemia, lymphoma and myeloma.
Regenerative medicine also has the potential to treat:
- Many other types of cancer: breast cancer, lung cancer, melanoma, kidney cancer, testicular cancer
- Heart disease: heart attack, heart failure
- Neurological disorders: Parkinson's disease, Alzheimer's disease, stroke, spinal cord injuries
- Eye diseases: macular degeneration, glaucoma, corneal defects
- Autoimmune diseases: diabetes, scleroderma, lupus erythematosus, rheumatoid arthritis, kidney disease
- Musculoskeletal disorders: cartilage injuries, osteoarthritis, muscular dystrophy
For more information, see Latest therapies.
How do discoveries go from bench to bedside?
Did you know that a therapy has to go through multiple research stages that attest to its efficacy and safety before it receives authorization? In fact, it takes a long time for a scientific discovery to become a treatment that doctors can prescribe to patients. Below is a short description of each research phase to give you a better idea of this rigorous process.
- Research and Development
Researchers test their ideas and hypotheses in a lab.
- Preclinical Research
In vitro or animal tests are done in a laboratory to demonstrate that a theory works in practice.
Before the therapy moves to the clinical trial stage (when it is administered to humans for the first time), Health Canada ensures that all tests demonstrating the therapy's relevance and safety have been conducted.
- Clinical Trials
- Phase I: Researchers evaluate tolerance and make sure there are no adverse effects in humans (i.e., they do not test the efficacy of the treatment). Subjects are generally healthy volunteers who are compensated.
- Phase II: Researchers identify the optimal dose at a small and intermediate scale. Patients may be involved at this stage
- Phase III: Researchers look to identify the therapy's true efficacy by comparing the results from these trials to results from regular therapies or placebo. At the end of Phase III, the therapy may or may not receive approval.
- Phase IV: After the market release of the therapy, researchers study its long-term effects and its effects on initially excluded populations.
CellCAN network affiliates conduct Research and Development, Preclinical Research, and Phase I to Phase III Clinical Trials.
Beware of unproven therapies
Many cell therapies are indeed available on the market (see Latest therapies), but you should be aware that a lot of stem cell research is still being tested in laboratories and early clinical trials.
Despite this fact, many health clinics around the world offer stem cell therapies through direct advertising to consumers, particularly over the Internet.
These therapies are generally sold at exorbitant prices, and no evidence-based data attests to their efficacy. What's worse, they can pose real risks for patients, for example, through the contamination of cells.
These clinics deliberately exploit vulnerable people by giving them misleading or insufficient information about the efficacy and potential risks of the therapy.
How can you recognize frauds?
These clinics are often in countries where legislation does not sufficiently protect patients. They tend to emphasize the benefits and omit the risks, provide personal testimonials instead of proof of efficacy, and refuse to provide scientific publications with the pretext that they are developing patents.
True clinical trials are registered, have strict admission criteria, and have received approval from regulatory agencies and ethics review boards. You are also consistently informed of how the clinical trial was designed, of any potential risks, and of any relevant alternatives. If you have trouble getting this information, then be wary. Above all, talk to your doctor!